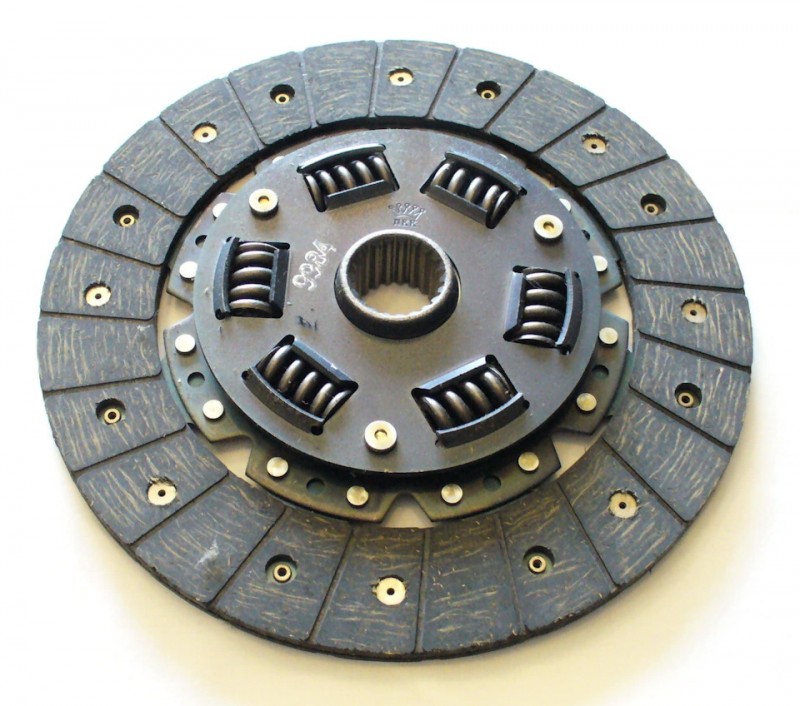
Диски сцепления ASCO для корейских грузовых автомобилей: выбор интернет-магазина
Качественные комплектующие — залог надежности и долговечности любого грузового автомобиля. В частности, диски сцепления играют ключевую роль в безотказной работе
Read More